- Home
- About
-
Solutions
-
Research Management
-
'%3E%3Crect x='0' y='0' width='1024' height='1024' fill='%231a222f'/%3E%3Cg transform='scale(4.00) translate(0.5 0.5)'%3E%3Crect fill='%23ffffff' fill-opacity='0.50' x='107' y='0' width='126' height='18'/%3E%3Cg transform='translate(87.79 248.56) rotate(90.73) scale(12.22 65.69)'%3E%3Cellipse fill='%23ffffff' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Crect fill='%2399ffff' fill-opacity='0.50' x='0' y='60' width='19' height='171'/%3E%3Crect fill='%239cffff' fill-opacity='0.50' x='239' y='16' width='17' height='180'/%3E%3Cg transform='translate(192.06 219.72) rotate(280.43) scale(29.96 174.27)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cg transform='translate(98.31 64.94) rotate(-59.89) scale(50.83 115.55)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cpolygon fill='%23aaf1cc' fill-opacity='0.50' points='238.79,8.33,271.00,89.16,226.23,24.64,33.83,-16.00'/%3E%3Cpolygon fill='%23aef3bd' fill-opacity='0.50' points='175.89,238.00,46.86,239.34,-16.00,200.81,41.86,271.00'/%3E%3Cellipse fill='%2301001a' fill-opacity='0.50' cx='151' cy='103' rx='86' ry='86'/%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='224' cy='165' rx='14' ry='143'/%3E%3C/g%3E%3C/g%3E%3C/svg%3E) Fund IQ | Research Project and Grant Management Solution
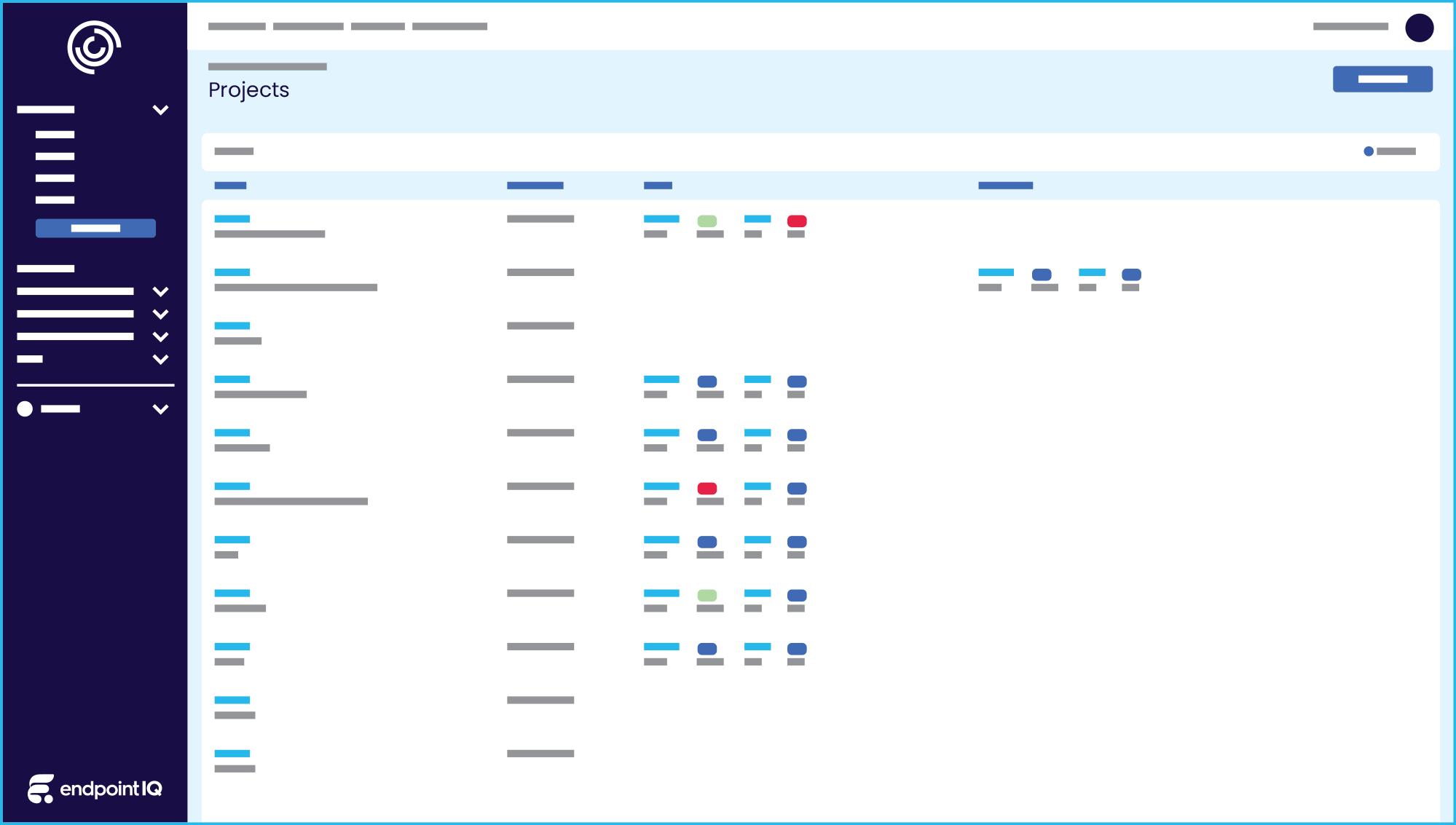
All your project types in one complete package
Fund IQ | Research Project and Grant Management Solution
All your project types in one complete package
-
'%3E%3Crect x='0' y='0' width='1024' height='1024' fill='%231a222f'/%3E%3Cg transform='scale(4.00) translate(0.5 0.5)'%3E%3Cg transform='translate(166.64 7.83) rotate(0.63) scale(64.47 13.11)'%3E%3Cellipse fill='%23ffffff' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cg transform='translate(9.49 143.88) rotate(179.81) scale(11.17 91.92)'%3E%3Cellipse fill='%2393ffff' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cpolygon fill='%23fefffa' fill-opacity='0.50' points='-5,229 175,244 71,271'/%3E%3Crect fill='%239fffff' fill-opacity='0.50' x='239' y='23' width='17' height='174'/%3E%3Cg transform='translate(146.86 213.59) rotate(102.83) scale(31.16 133.32)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cg transform='translate(104.72 53.47) rotate(286.08) scale(39.23 140.65)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cg transform='translate(231.84 18.85) rotate(37.64) scale(20.80 9.38)'%3E%3Cellipse fill='%23c5ffff' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cg transform='translate(147.07 126.34) rotate(96.95) scale(108.11 90.54)'%3E%3Cellipse fill='%23000013' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Crect fill='%23d2ffb8' fill-opacity='0.50' x='77' y='238' width='74' height='18'/%3E%3Crect fill='%23bdffb5' fill-opacity='0.50' x='105' y='0' width='111' height='18'/%3E%3C/g%3E%3C/g%3E%3C/svg%3E) Impact IQ | Research Tracking and Impact
All-in-one system for research tracking and impact
Impact IQ | Research Tracking and Impact
All-in-one system for research tracking and impact
-
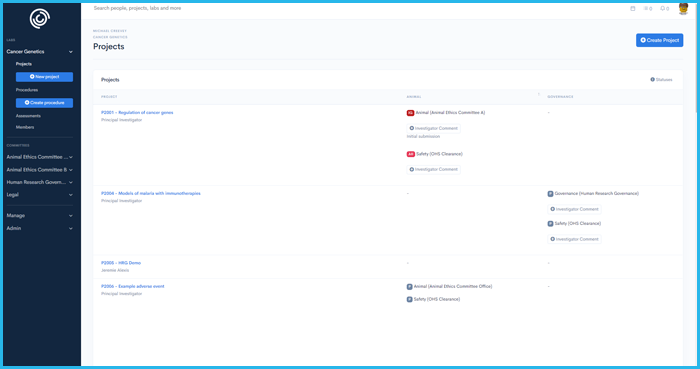
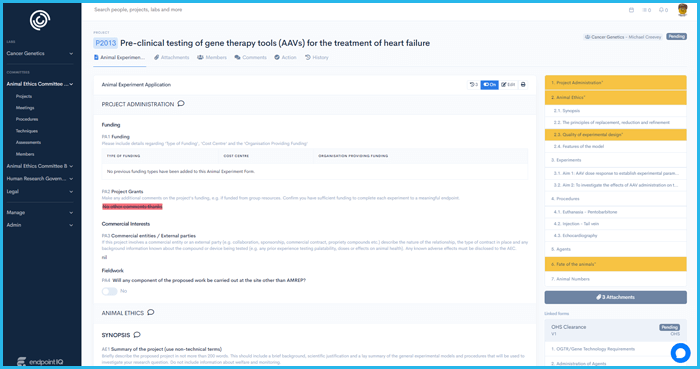
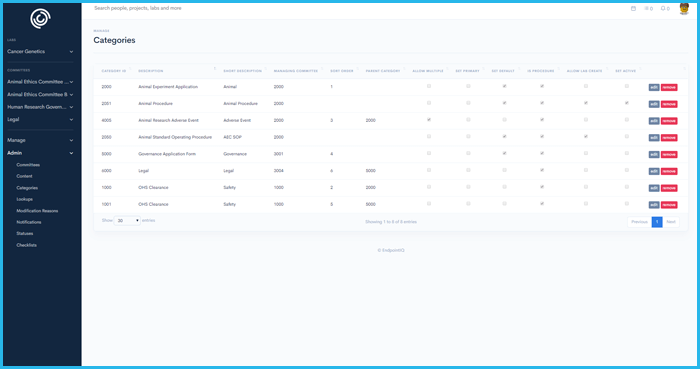
'%3E%3Crect x='0' y='0' width='1024' height='1024' fill='%231a222f'/%3E%3Cg transform='scale(4.00) translate(0.5 0.5)'%3E%3Cellipse fill='%23ffffff' fill-opacity='0.50' cx='165' cy='9' rx='69' ry='11'/%3E%3Cg transform='translate(90 255) rotate(0) scale(120 34)'%3E%3Crect fill='%23ffffff' fill-opacity='0.50' x='-0.5' y='-0.5' width='1' height='1'/%3E%3C/g%3E%3Cellipse fill='%2399ffff' fill-opacity='0.50' cx='8' cy='144' rx='11' ry='94'/%3E%3Cg transform='translate(247.63 108.10) rotate(270.34) scale(94.33 10.21)'%3E%3Cellipse fill='%2395ffff' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cellipse fill='%23000005' fill-opacity='0.50' cx='160' cy='94' rx='78' ry='78'/%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='53' cy='26' rx='55' ry='55'/%3E%3Cpolygon fill='%23beffff' fill-opacity='0.50' points='-4,190 19,246 54,255'/%3E%3Cg transform='translate(178.35 212.65) rotate(191.45) scale(164.78 34.59)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cpolygon fill='%23c1fffe' fill-opacity='0.50' points='238,10 191,2 260,48'/%3E%3Cpolygon fill='%23c2ffb2' fill-opacity='0.50' points='106,18 228,14 106,-16'/%3E%3C/g%3E%3C/g%3E%3C/svg%3E) Integrity IQ | Ethics and Governance
Ethics simplified, Research empowered
Integrity IQ | Ethics and Governance
Ethics simplified, Research empowered
-
'%3E%3Crect x='0' y='0' width='1024' height='1024' fill='%231a222f'/%3E%3Cg transform='scale(4.00) translate(0.5 0.5)'%3E%3Cg transform='translate(170 8) rotate(0) scale(126 21)'%3E%3Crect fill='%23ffffff' fill-opacity='0.50' x='-0.5' y='-0.5' width='1' height='1'/%3E%3C/g%3E%3Crect fill='%23ffffff' fill-opacity='0.50' x='21' y='238' width='131' height='18'/%3E%3Cellipse fill='%2399ffff' fill-opacity='0.50' cx='9' cy='145' rx='11' ry='91'/%3E%3Cellipse fill='%239cffff' fill-opacity='0.50' cx='248' cy='109' rx='11' ry='92'/%3E%3Cpolygon fill='%23000000' fill-opacity='0.50' points='271,237 45,238 14,139'/%3E%3Cg transform='translate(76.13 40.90) rotate(189.22) scale(165.02 30.92)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3Cellipse fill='%23000017' fill-opacity='0.50' cx='145' cy='131' rx='95' ry='95'/%3E%3Cpolygon fill='%23acf5ec' fill-opacity='0.50' points='-7.70,189.07,54.65,254.90,20.02,247.74,-16.00,187.62'/%3E%3Cpolygon fill='%23b1faf8' fill-opacity='0.50' points='244,14 210,0 261,73'/%3E%3Cg transform='translate(204.53 222.77) rotate(279.79) scale(38.64 73.36)'%3E%3Cellipse fill='%23000000' fill-opacity='0.50' cx='0' cy='0' rx='1' ry='1'/%3E%3C/g%3E%3C/g%3E%3C/g%3E%3C/svg%3E) Scholar IQ | Graduate Research Student Management
Candidature lifecyle management
Scholar IQ | Graduate Research Student Management
Candidature lifecyle management
-
-
contact
- Support
BOOK A DEMO
BOOK A DEMO